Mode of Action
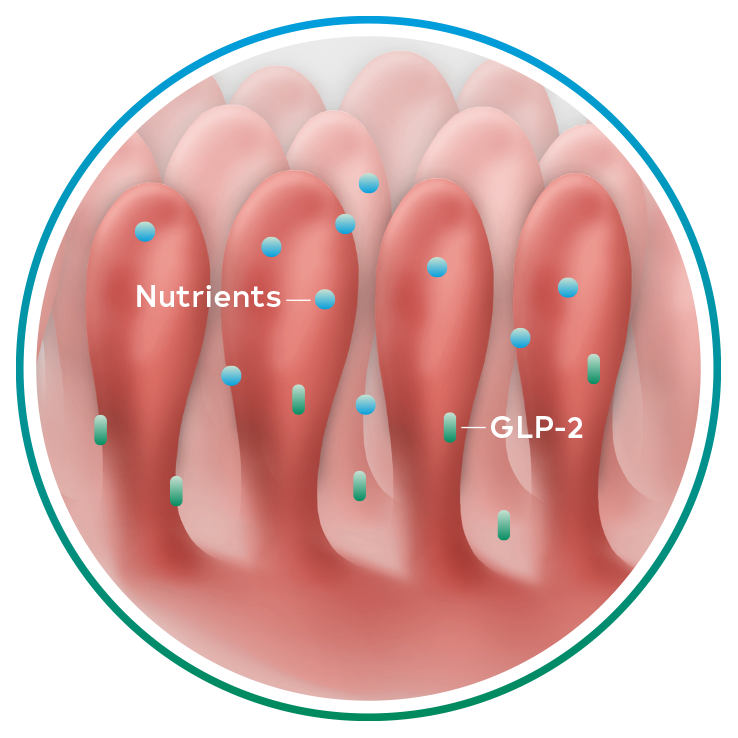
Revestive mimics naturally occurring GLP-2 and results in physiologically enhanced absorption in patients with short bowel syndrome-intestinal failure1-3
Naturally occurring GLP-2, an intestinal hormone, plays an important role in maintaining the normal structure and function of the small intestine and in the absorption of nutrients4-6
GLP-2 secretion1,4-6
Secretion of naturally occurring GLP-2 is significantly impaired in patients with short bowel syndrome-intestinal failure (SBS-IF) compared to non-SBS patients7
Revestive is a recombinant analogue of human GLP-2 with a longer half-life than naturally occurring GLP-21
In patients with SBS-IF, Revestive:1,3
- Physiologically enhanced absorption
- Increased intestinal villus height and crypt depth vs baseline*
- Stimulated intestinal rehabilitation and increased the absorptive capacity of the intestine**
Revestive increased intestinal villus height and crypt depth vs. baseline3
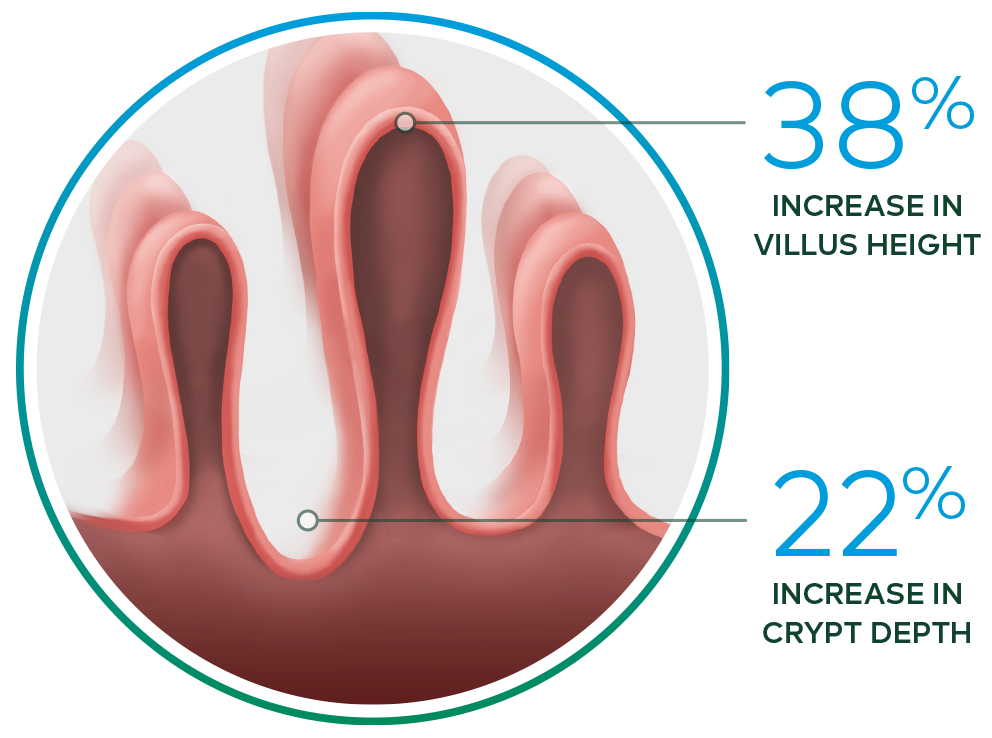
*In a 21-day, Phase II, open-label, multicentre, dose-ranging study evaluating the safety and effect of Revestive in 17 patients with SBS-IF, treatment with Revestive at doses from 0.03 to 0.15 mg/kg/day in single or divided doses. 8 of 10 patients with end jejunostomy were biopsied and found to have increased intestinal villus height and crypt depth, compared with baseline.
** Treatment resulted in enhanced gastrointestinal fluid absorption of approximately 750-1000 mL/day with improvements in the absorption of electrolytes.
GLP-2= glucagon-like peptide-2; SBS-IF= short bowel syndrome – intestinal failure
References
- Revestive (teduglutide) Summary of Product Characteristics. Shire Pharmaceuticals Ireland Limited, Dublin, Ireland. November 2017.
- Hofstetter S, Stern L, Willet J. Key issues in addressing the clinical and humanistic burden of short bowel syndrome in the US. Curr Med Res Opin. 2013;29:495-504.
- Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224-1231.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care. 2009;12:526-532.
- Tee CT, Wallis K, Gabe SM. Emerging treatment options for short bowel syndrome: potential role of teduglutide. Clin Exp Gastroenterol. 2011;4:189-196.
- Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561-583.
- Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure Gut. 1999;45:559-563.