Pediatric Patients
Short Bowel Syndrome
Short Bowel Syndrome with intestinal failure (SBS-IF) is defined as the reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such that intravenous (IV) supplementation is required to maintain health and/or growth.1
In children
SBS-IF is most commonly caused by congenital diseases including:2-5
- Necrotising enterocolitis
- Midgut volvulus
- Intestinal atresia
- Intestinal aganglionosis
- Gastroschisis
- Malrotation
- Trauma
- Hirschsprung disease
Long-term parenteral nutrition/IV fluids (PN/IV) is associated with severe complications and can affect patient’s quality of life6,7
What if you could free your paediatric patients with short bowel syndrome-intestinal
failure (SBS-IF) from parenteral nutrition/IV fluids?
It may be possible
with Revestive8
Revestive is the first and only long-term therapy
indicated for the treatment of patients with SBS-IF.8-11
Efficacy
Children with short bowel syndrome-intestinal failure
In children with SBS-IF*, Revestive reduced PN/IV requirements11
REDUCED PN/IV VOLUME REQUIREMENTS8,11
At 24 weeks, 69% of patients treated with Revestive at the approved dose (0.05 mg/kg/day) achieved at least a 20% reduction from baseline in weekly PN/IV volume (n=26 patients)1,11
- vs 11% with standard of care**
REDUCED THE NUMBER OF DAYS/WEEK ON PN/IV***8,11
- Mean reduction in PN/IV by 1.3 days/week†1,11
GAVE FREEDOM FROM PN/IV^^
- As early as Week 24, 3/26 children treated
with Revestive were independent from PN/IV†1,11
– vs. 0 with standard of care
IN ADDITION, AT WEEK 24 REVESTIVE REDUCED MEAN PN/IV VOLUME‡11
- Mean reduction in PN/IV volume of 42% at Week 24†11
Mode of Action
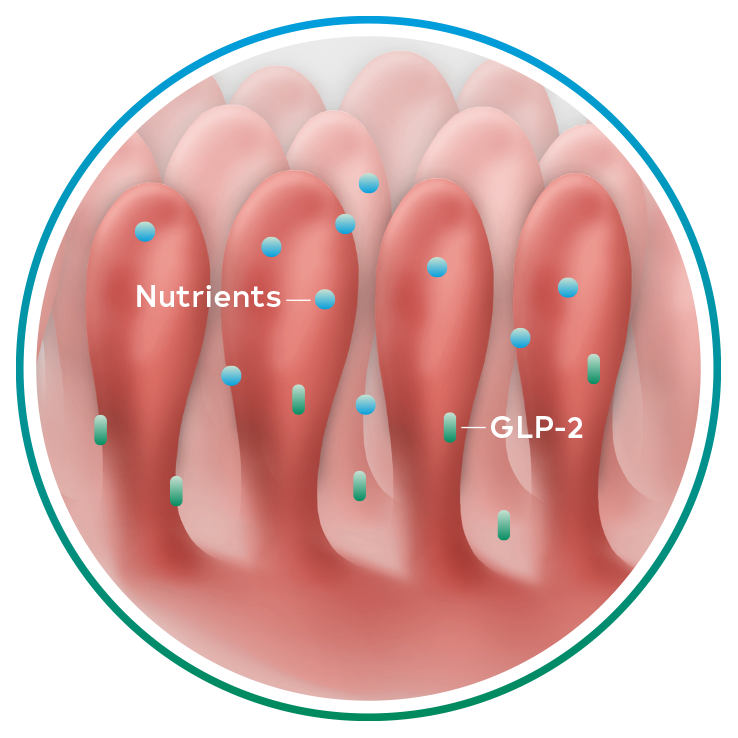
Naturally occurring GLP-2, an intestinal hormone, plays an important role in maintaining the normal structure and function of the small intestine and in the absorption of nutrients12-14
GLP-2 secretion8,12-14
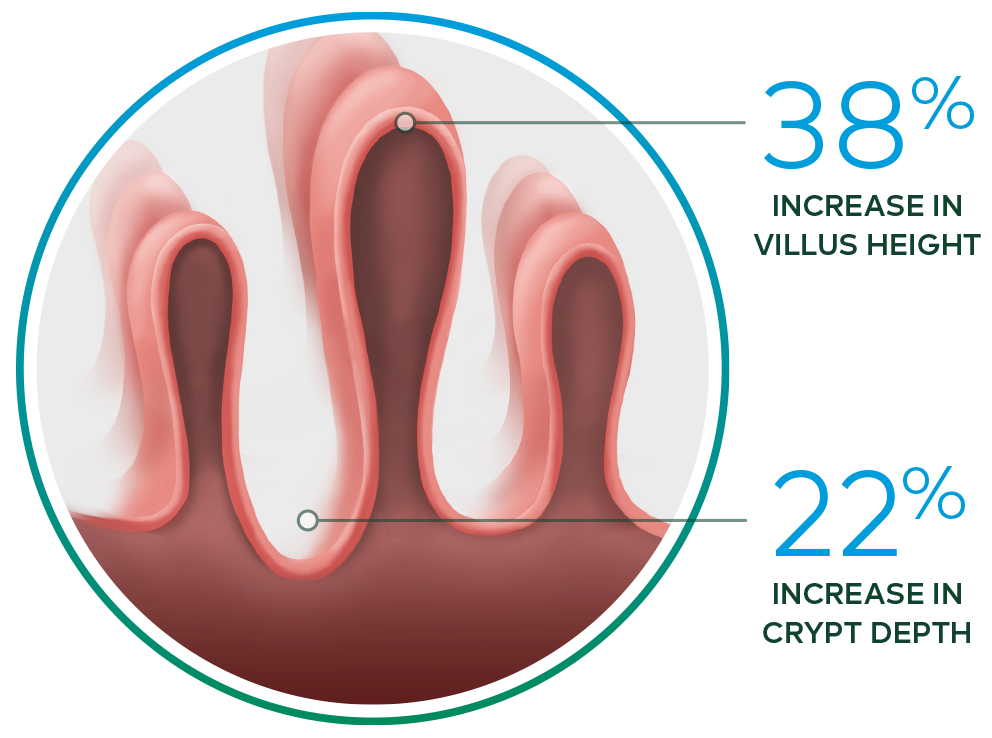
Revestive increased intestinal villus height and crypt depth vs. baseline§ 13-15
Safety
Revestive was well tolerated across multiple clinical trials in adults
and children with short bowel syndrome intestinal
failure8
Children
In two completed clinical trials§ in children aged 1–17 years-old with short bowel syndrome-intestinal failure treated with Revestive for up to 6 months:8
- No patient discontinued due to adverse events
- Most treatment-emergent adverse events were mild to moderate in severity
- The overall safety profile (type and frequency of events) was similar to that reported in adults
No new safety signals seen in long-term pooled paediatric data^ of 89 patients treated with Revestive for 51.7 weeks (median; range 5.0–94.7) and followed up for 83 weeks16
*Revestive was studied in a 12-week, open-label, clinical study in 42 paediatric patients aged 1 year through 14 years with SBS who were dependent on PN/IV. Three (3) doses of Revestive, 0.0125 mg/kg/day (n=8), 0.025 mg/kg/day (n=14), and 0.05 mg/kg/day (n=15), were investigated for 12 weeks. Five (5) patients were enrolled in an SOC cohort. An additional 24-week, randomised, double-blind, multicentre study was conducted in 59 paediatric patients aged 1 year through 17 years who were dependent on PN/IV. Two (2) doses of Revestive were studied: 0.025 mg/kg/day (n=24) and 0.05 mg/ kg/day (n=26); nine (9) patients were enrolled in an SOC arm.
^Safety data of 89 paediatric patients aged 1-17 years-old treated with REVESTIVE from 4 clinical trials were pooled. Data from the completed 12-week and 24-week clinical trials* and interim data from ongoing open-label extension studies to 12-week and 24-week trials were included. The study objective was to assess the safety of REVESTIVE in children with SBS-IF including when used beyond 24-weeks.
Dosing and administration8
Revestive is given by subcutaneous injection once daily.
The recommended dose is 0.05 mg/kg.
Two vial sizes are available:
- 5.0 mg per vial
- 1.25 mg per vial (an option for children who weigh less than 20 kg)
Vials should be reconstituted with the supplied solvent (0.5 mL).
The reconstituted solution should be administered by subcutaneous injection once daily, alternating sites between 1 of the 4 quadrants of the abdomen. In case the injection into the abdomen is hampered by pain, scarring or hardening of the tissue, the thigh can also be used.
Revestive should not be administered intravenously or intramuscularly.
If a dose is missed, that dose should be injected as soon as possible on that day.
Children8
Dosing table: 1.25 mg vial
| BODY WEIGHT | 1.25 mg/0.5 mL Volume to be injected |
|---|---|
| 5-6 kg | 0.10 ml |
| 7-8 kg | 0.14 ml |
| 9-10 kg | 0.18 ml |
| 11-12 kg | 0.22 ml |
| 13-14 kg | 0.26 ml |
| 15-16 kg | 0.30 ml |
| 17-18 kg | 0.34 ml |
| 19-20 kg | 0.38 ml |
| >20 kg | Use the 5mg strength vial |
Dosing table: 5.0 mg vial
| BODY WEIGHT | 5 mg/0.5 mL Volume to be injected |
|---|---|
| 10-11 kg | 0.05 ml |
| 12-13 kg | 0.06 ml |
| 14-17 kg | 0.08 ml |
| 18-21 kg | 0.10 ml |
| 22-25 kg | 0.12 ml |
| 26-29 kg | 0.14 ml |
| 30-33 kg | 0.16 ml |
| 34-37 kg | 0.18 ml |
| 38-41 kg | 0.20 ml |
| 42-45 kg | 0.22 ml |
| 46-49 kg | 0.24 ml |
| ≥50 kg | See “Adults” section |
A treatment period of 6 months is recommended after which treatment effect should be evaluated. In children below the age of two years, treatment should be evaluated after 12 weeks. There are no data available in paediatric patients after 6 months.
*Children with SBS-IF aged 1 – 17 (<18) years, all patients were unable to reduce their PN/IV dependence for at least 3 months prior to study entry.
** Weight normalised reduction.
*** Standard deviation for Revestive: -1.3±2. 6.6 days/week baseline PN/IV requirement for Revestive study population, 6.6 days/week baseline PN/IV requirement for SOC study population.
† Patients treated with Revestive 0.05 mg/kg/day.
‡ Standard deviation for Revestive: -41.6±28.90; SOC: -10.2±13.5926.
§ In a 21-day, Phase II, open-label, multicentre,
dose-ranging study evaluating the safety and effect of Revestive in 17 patients with SBS-IF,
treatment with Revestive at doses from 0.03 to 0.15 mg/kg/day in single or divided doses. 8 of
10 patients with end jejunostomy were biopsied and found to
have increased intestinal villus
height and crypt depth, compared with baseline.
GLP-2= glucagon-like peptide-2; SBS-IF= short bowel syndrome – intestinal failure
^^3/26 paediatric patients stable on their PN/IV for at least 3 months at baseline and treated with REVESTIVE completely weaned from PN/IV by Week 24 vs 0/9 patients on SoC 8,11
References
- Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247-307
- Chandra R, Kesavan A. Current treatment paradigms in pediatric short bowel syndrome. Clin J Gastroenterol. 2018;11:103-112.
- Wales PW, Christison-Lagay ER. Short bowel syndrome: epidemiology and etiology. Semin Pediatr Surg. 2010;19:3-9.
- Buchman AL. In: Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Pathophysiology/Diagnosis/Management. 10th ed. Philadelphia, PA: Saunders Elsevier; 2016:1832-1848.
- Duggan CP, Jaksic T. Pediatric intestinal failure. N Engl J Med. 2017;377:666-675.
- Hofstetter S, Stern L, Willet J. Key issues in addressing the clinical and humanistic burden of short bowel syndrome in the US. Curr Med Res Opin. 2013;29:495-504.
- Huisman-de Waal G, Schoonhoven L, Jansen J, et al. The impact of home parenteral nutrition on daily life-a review. Clin Nutr. 2007;26:275-288.
- Revestive (teduglutide) Summary of Product Characteristics. Shire Pharmaceuticals Ireland Limited, Dublin, Ireland. November 2017.
- Schwartz LK, O’Keefe SJD, Fujioka K, et al. Long-term safety and efficacy of teduglutide for the treatment of intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7:e142.
- Shire Press Release. 2016.
- Kocoshis SA, Merritt RJ, Hill S, et al. Safety and Efficacy of Teduglutide in Pediatric patients With Intestinal Failure due to Short Bowel Syndrome: A 24-Week, Phase III Study. JPEN J Parenter Enteral Nutr. 2020;44(4):621-631.
- Wallis K, Walters JR, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care. 2009;12:526-532.
- Tee CT, Wallis K, Gabe SM. Emerging treatment options for short bowel syndrome: potential role of teduglutide. Clin Exp Gastroenterol. 2011;4:189-196.
- Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561-583.
- Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224-1231.
- Hill S, Carter BA, Cohran V, et al. Safety Findings in Pediatric Patients During Long-Term Treatment With Teduglutide for Short-Bowel Syndrome–Associated Intestinal Failure: Pooled Analysis of 4 Clinical Studies JPEN . J Parenter. Enteral Nutr 2020 https://doi.org/10.1002/jpen.2061 E-pub ahead of print